Isotopes & Dating in Marine Environments
Dating
There are numerous different options for dating marine samples, each with applicable timespans, levels of certainty and limitations.
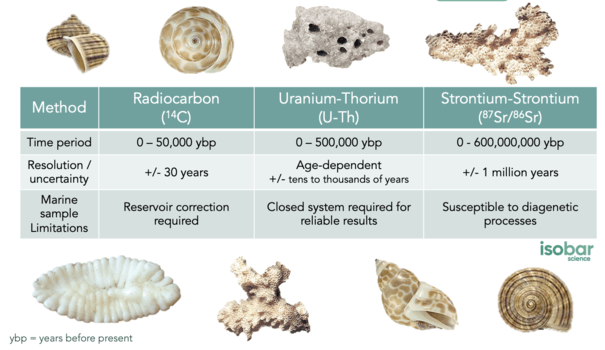
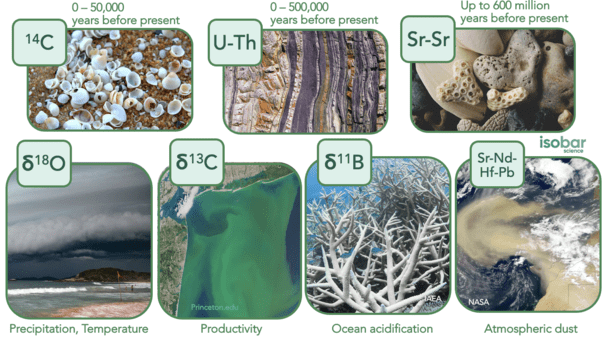
Radiocarbon (14C) Dating is thought to be one of the most accurate dating methods for terrestrial samples, developed from high resolution tree-rings from the north and south hemispheres. However, this method can be challenging when applied to marine samples due to the marine reservoir effect. The ocean has a long overturning rate – sometimes on the order of 1000 years or more – meaning that many regions within the ocean are not at equilibrium with the atmosphere, resulting in offsets between atmospheric and oceanic 14C. As a result, the 14C measurements of marine samples can result in samples being dated significantly older than in reality. To resolve this, a marine reservoir collection must be applied to all marine samples dated with this methodology. Regional to local correction factors are preferred to reduce dating range errors. This methodology can be applied to samples from the last 40,000 – 50,000 years with a best case error of +/- 20-30 years.
Uranium Thorium (U-Th) Dating can be utilized to date samples ranging from present day to 500,000 years before present, making this method applicable across a far greater timespan than radiocarbon dating. However, this method is most reliable for samples aged 200 – 450,000 years. It is based on the decay of the parent isotopes (uranium-234) and the concurrent production of the daughter isotope (thorium-230). The certainty of this dating method is highly dependent on the specific time period, with uncertainties ranging from tens to thousands of years. Samples must also be considered a closed system with regard to uranium and thorium to yield accurate results such as corals; whereas those considered an open system are more likely to result in erroneous dates (including foraminifera, bones and shells).
Strontium-Strontium (87Sr/86Sr) Dating is another method applied even further back in time – from present day to 600 million years ago with errors of +/- 1 million years. However, it tends to be most accurate from 500,000 years of age onwards. Dating with strontium is based on the fact that the ratio 87Sr/86Sr is a function of continental weathering which varies through time. Such variations have been established through time using foraminifera, belemnite guards, ammonite aragonite, atoll carbonates and marine cement to develop the Seawater Strontium Curve. While the uncertainty of strontium dating can be quite high with this 1 million year resolution, it is one of only a few methods available for samples that fall beyond the time thresholds of radiocarbon and U-Th dating.
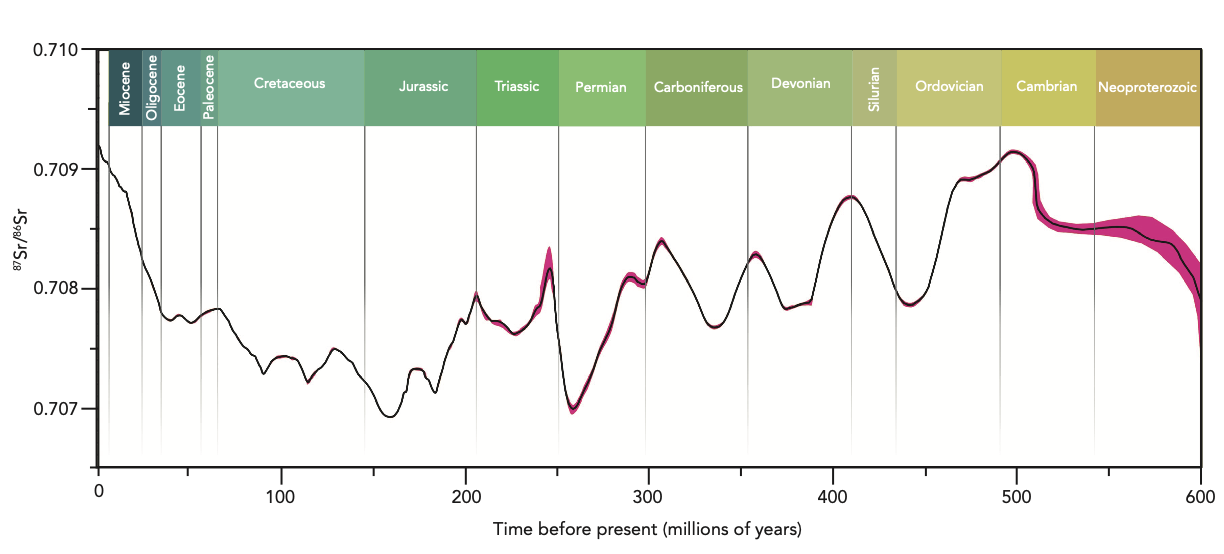
The seawater strontium curve used to calibrate and date marine samples
Reconstructing climate and marine environments
A variety of different isotopes can be applied to marine samples in order to reconstruct past climate and marine environmental conditions through time.
Stable oxygens (δ18O) and carbon isotopes (δ13C) are two of the most common isotopes used on marine samples. As two very important elements in marine environments, they can be utilized to reconstruct many different variables, depending on the type of sample and sample location. For example – when applied to foraminifera, δ18O can provide information on the ocean temperature and precipitation (globally for benthic and locally for planktonic) as well as ice volume. Whereas δ13C provides more information on oceanic transportation and circulation, including the age of bottom waters and level of continental weathering for benthic forms, in addition to the level of local productivity and upwelling for planktonic varieties. δ18O and δ13C are also commonly measured in coral samples to reconstruct climate variability including recent ocean warming and phases of El Niño / La Niña.
Another common isotope in marine studies is boron as boron isotopes (δ11B) are an important variable in the reconstruction of past ocean conditions due to the correlation between fractionation of δ11B, oceanic pH and CO2. This relationship is particularly important in reconstructing the trends in ocean acidification in both recent time due to anthropogenic climate as well as in deep time – including the PETM extinction event (Paleocene-Eocene Thermal Maximum).
One final series of isotopes commonly used are lead (Pb), neodymium (Nd), hafnium (Hf) and strontium (Sr), which are inherent in many geological settings. Together and separately, these isotopes have been used to trace the evolution of metamorphic and igneous rocks, track the origin of sediments and dust, analyze weathering regimes and reconstruct past ocean circulation.
| Application | Isotope | Relevant Marine Samples |
| Dating | Radiocarbon (14C) | Marine mammal bones, fish otolith, foraminifera, organic sediment, pteropods, shell, coral, carbonates, limestone |
| Uranium-Thorium (U-Th) | Coral, shell*, bone*, foraminifera*, marine cements, organic sediment*, carbonates | |
| Strontium-Strontium (87Sr/86Sr) | Foraminifera, shells, corals, carbonates | |
| Environmental / Climate Reconstruction | Oxygen (δ18O) | Marine mammal bones, fish otolith, foraminifera, organic sediment, pteropods, shell, coral, carbonates, limestone |
| Carbon (δ13C) | Marine mammal bones, fish otolith, foraminifera, organic sediment, pteropods, shell, coral, carbonates, limestone | |
| Boron (δ11B) | Shells, coral, carbonates |
*less optimal for accurate results
**Strontium (Sr) only
Isobar Science discontinued the Nd-Hf service starting January 2024.
