Boron Isotopes (B) Geochemistry Overview
Boron has two forms of stable isotopes (10B, 11B) as well as 13 forms of radioactive isotopes (ranging from 7B through to 21B, not including the stable forms). Stable forms of boron are the only naturally occurring isotopes with 10B making up 20% of natural boron and 11B making up 80%.
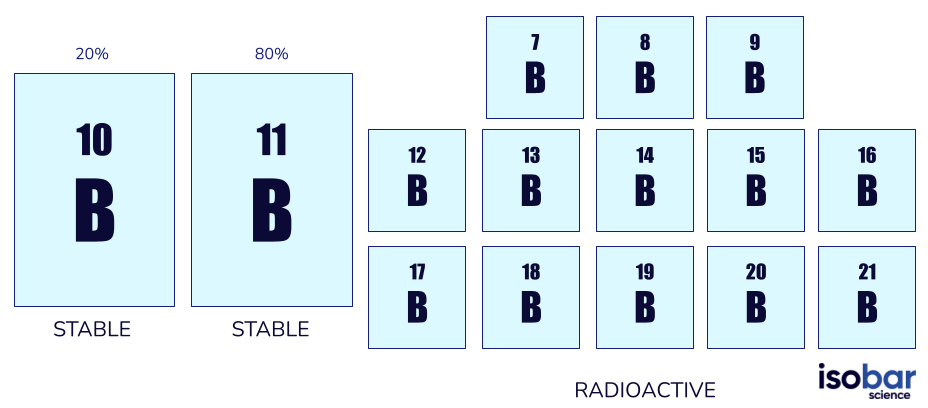
The two boron isotope species that are commonly used in geochemical studies (left) and the further 13 radioactive boron isotope species (right)
Different earth system materials and environmental conditions are characterized by discrete ranges for the boron isotopic ratio. Thus, this parameter is extensively used in geochemical fingerprinting, source tracking, contamination prediction, global carbon cycles, and ocean circulation studies.
More information on Sample Types and Selection for boron analysis
Disclaimer: This video is hosted in a third-party site and may contain advertising.
This video excerpt is part of Beta Analytic’s webinar: Boron Isotopic Analysis
Boron Isotopes in Paleoclimatology & Paleoceanography
Dissolved boron in seawater primarily appears in the form of two mononuclear species, boric acid (H3BO3 or B(OH)3) and borate (BO3-3 or B(OH)4−), with a distinct isotopic fractionation between the two species such that boric acid is enriched in the heavier isotope (11B) by 27.2%. The relative proportion of these species is pH dependent as shown below:
B(OH)3 + H2O ↔ B(OH)4− + H+
High pH ↔ Low pH
Boron is incorporated into biogenic and inorganic carbonates primarily as the borate species. However, the particular species and concentration of boron incorporated into biogenic carbonates (e.g. shells) is pH dependent. Thus, the boron isotopic composition of marine biogenic carbonates reflects seawater pH.
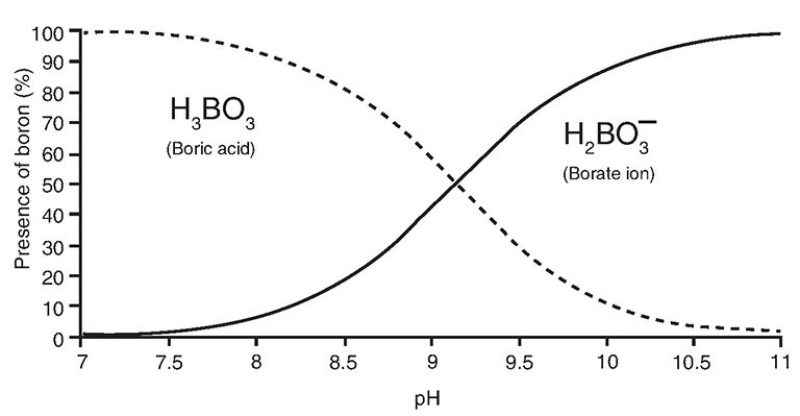
The relationship between pH and the percentage of two different boron species (https://jpt.spe.org/comparison-methods-boron-removal-flowback-and-produced-waters)
Read more on our blog posts:
Geochemical Fingerprinting and Contaminant Source Tracking
Boron is widely used in industry (lubricant, flux, additive), agriculture (micronutrient in fertilizers), and households (bleaching agent). Boron is an important additive in steel manufacturing to increase the hardenability of a material. For example, boron steels are widely used in the nuclear industry to shield systems and control nuclear reactions given its natural ability to absorb neutrons. In other industries, a boron compound known as sodium borate (“Borax”) is used extensively for detergents to remove stains and mildew as well as in cosmetic products as a preservative and buffering agent.
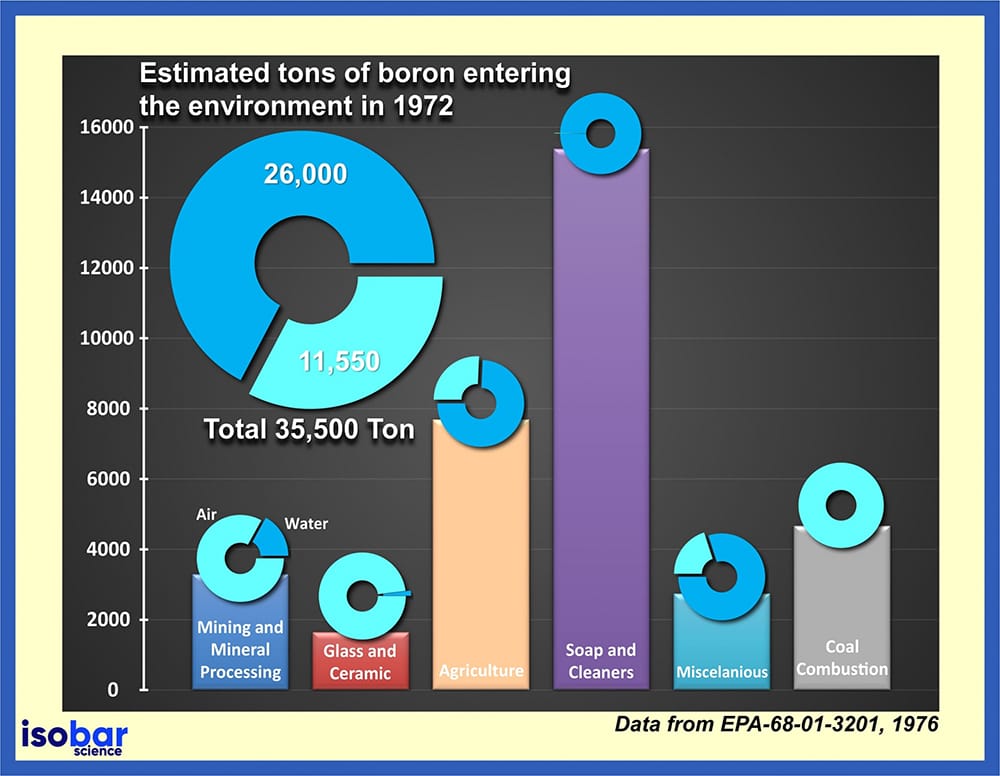
Estimated tons of boron entering the environment since 1972 (Data from EPA-68-01-3201,1976).
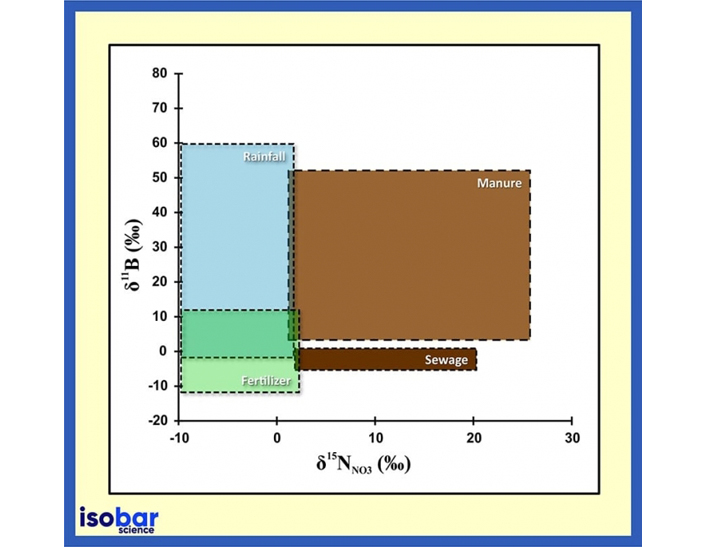
Source signature of boron and nitrogen isotopic ratios in water (Briand et al., 2013).
Given the extensive use of boron in many different products, it is released to the environment on a regular basis. Based on data provided by the US Environmental Protection Agency (EPA, 1976), in 1972 alone, a total of 35.5 kilotons of boron was released into the environment, 73% of which was directly introduced to the water. The release of boron is particularly problematic as boron cannot be destroyed in the environment, but rather it can change its form by separating and becoming attached to other particles in soils, sediments, and water. This boron can eventually make its way into food (vegetables and fruit) given its an essential nutrient for plant growth.
References
Briand, C., Plagnes, V., Sebilo, M., Louvat, P., Chesnot, T., Schneider, M., Ribstein, P. and Marchet, P., (2013). Combination of nitrate (N, O) and boron isotopic ratios with microbiological indicators for the determination of nitrate sources in karstic groundwater. Environmental Chemistry, 10(5), pp.365-369. DOI: 10.1071/EN13036
Chalk, T.B., Foster, G.L. and Wilson, P.A., (2019). Dynamic storage of glacial CO2 in the Atlantic Ocean revealed by boron [CO32−] and pH records. Earth and Planetary Science Letters, 510, pp.1-11. DOI: 10.1016/j.epsl.2018.12.022
EPA (Environmental Protection Agency), (1976). Chemical technology and economics in environmental perspectives. Task II- Removal of Boron from wastewater. Environmental Protection Agency, Office of Toxic Substances, Washington, D.C. 20460
Giri, S.J., Swart, P.K. and Pourmand, A., (2019). The influence of seawater calcium ions on coral calcification mechanisms: Constraints from boron and carbon isotopes and B/Ca ratios in Pocillopora damicornis. Earth and Planetary Science Letters, 519, pp.130-140. DOI: 10.1016/j.epsl.2019.05.008
Martínez-Botí, M.A., et al. (2015). Boron isotope evidence for oceanic carbon dioxide leakage during the last deglaciation. Nature, 518(7538), pp.219-222. DOI: 10.1038/nature14155
Page last updated: January 2020

