Geochemistry: Stable and Radioactive Isotopes
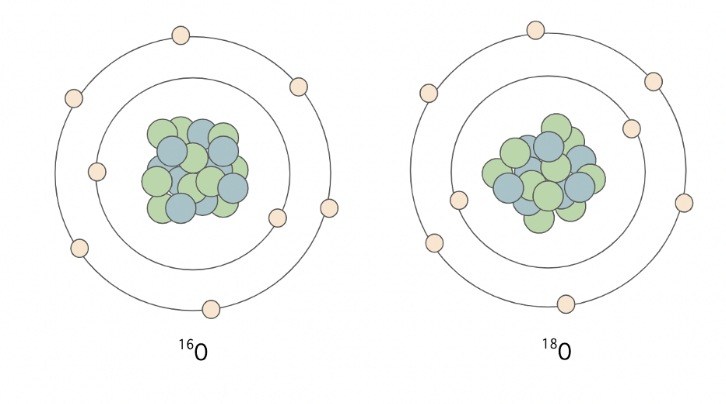
In geochemical research, stable and unstable isotopes are used to understand the chemistry behind natural processes. Isotopes are different forms of a single element, with differing numbers of neutrons within their nucleus, resulting in different atomic masses. For example – oxygen has many known isotopes, including the three most common ones: 16O, 17O and 18O. These forms of the same element move through the hydrosphere, geosphere and atmosphere differently as a result of partitioning of heavier and lighter forms (isotopic fractionation).
Isotopes can be stable or radioactive in nature, and differ in a variety of ways:
| Stable Isotopes | Radioactive Isotopes |
| Abundance in nature | Rare in nature |
| Do not emit radiation | Spontaneously emit radiation |
| Stable, constant atomic mass | Unstable, changing atomic mass |
| No half-life, or very long half-life | Short, detectable half-life |
UPDATE: Isobar Science discontinued the Sr-Nd-Hf service starting January 2024.
The delta (δ) notation is used when comparing the relative abundance of one isotope (e.g. 16O) compared to another isotope from the same elements (e.g. 18O), expressed in parts per thousand (per mil: ‰) and reported with respect to international standards (e.g. oxygen is reported against SMOW = Standard Mean Ocean Water).
Generally, stable isotopes are used in geochemical research when analysing the variability in a natural process (e.g. climate) where radioactivity is not occurring. Alternatively, radioactive isotopes are analysed when the decay is reflecting a natural process (e.g. decay of radiocarbon of dead organisms).
| Stable Isotope | Application Example |
| Boron Isotopes (δ11B) | Reconstructing oceanic pH (and ocean acidification) by analysing δ11B in coral growth layers |
| Oxygen Isotopes (δ18O) | Developing a timeline changes in precipitation type and origin by analysing δ18O in tree-ring cellulose |
| Carbon Isotopes (δ13C) | Analysing changes in vegetation ecosystems (C3 and C4 plant types) by measuring δ13C in speleothems |
| Nitrogen Isotopes (δ15N) | Investigating dietary patterns in organisms by analysing δ15N (and δ13C) in tooth enamel |
| Lead Isotopes (204Pb, 206Pb, 207Pb and 208Pb) | Analysing lead isotopes in ice core dust to decipher origin and variations in atmospheric circulation |
| Strontium Isotopes (87Sr/86Sr) | Reconstructing migration pathways of early civilizations by analysing changes in 87Sr/86Sr in human bones (early life analysis) and nails/hair (late life analysis) |
| Radiogenic Isotope | Application Example |
| Radiocarbon (14C) | Developing an age-depth curve to date lake sediment microfossils for paleoclimatological analysis |
| Strontium-Neodymium-Hafnium (Sr-Nd-Hf) | Analysing melting and magma evolution by measuring Sr-Nd-Hf systematics in rocks |
| Uranium-Thorium (U-Th) | Dating calcium carbonate organisms by analysing the decay and uranium and production of thorium |