Strontium Isotopes (87Sr/86Sr) for Geochronology
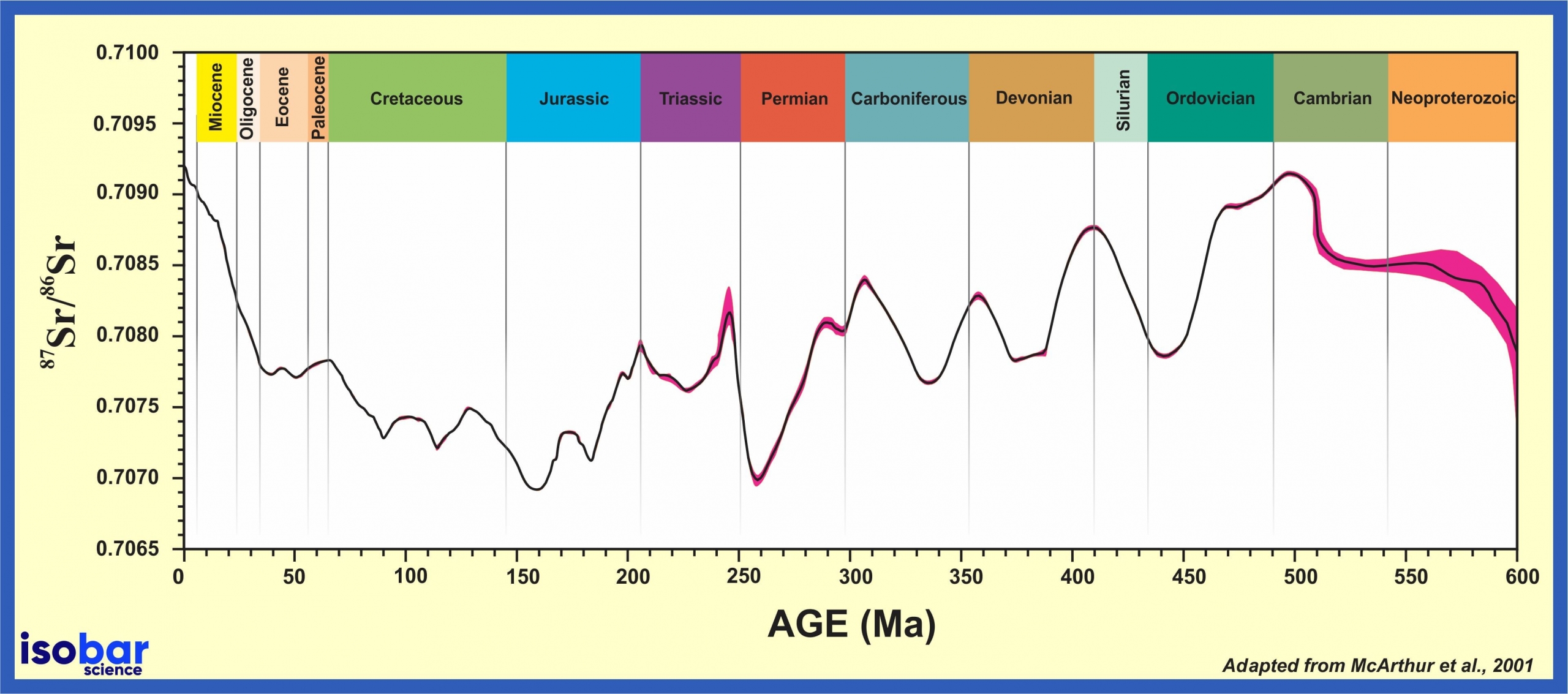
Strontium ratios (87Sr/86Sr) have varied in the world’s oceans through time as a result of fluctuations in strontium type and availability, often resulting from weathering of continental materials as well as volcanic activity at the mid-oceanic ridge. Such variations are recorded in oceanic sediment as a result of the precipitation of minerals from seawater, which has been used as a basis for the 87Sr/86Sr marine curve. This curve covers hundreds of millions of years and is used to date marine minerals/carbonates.
Reliability of Sr-Sr Dating over time
The certainty of 87Sr/86Sr dating is not consistent through time, with higher certainty ascribed to younger samples due to the higher abundance of younger samples to calibrate the curve. In order to improve certainty, rather than a one-to-one comparison of the 87Sr/86Sr value in a discrete sample compared to the 87Sr/86Sr curve, this dating method is more accurate when trends of 87Sr/86Sr in minerals are developed and compared to different sections of the Sr-Sr seawater curve. In these cases, optimal 87Sr/86Sr profiles are those that have a relatively stable sedimentation rate, very few if any stratigraphic gaps (e.g. unconformities) and strong variations in 87Sr/86Sr over time. Sample profiles with significant 87Sr/86Sr change over time can be more accurately dated compared to those with limited 87Sr/86Sr variability, as represented by plateaus through their stratigraphic sequences.
Sample types
Samples used for 87Sr/86Sr dating are generally macrofossil carbonates, foraminifera, brachiopods, trilobites and marine cements; whereas ammonites and bulk sediment have been found to be less accurate (Mearon et al. 2003; Peterman et al. 1970). Regardless of the sample type, a lack of diagenetic effects, strong preservation of the sample and accordingly a retention of the original 87Sr/86Sr ratio is required for successful dating. Alteration of the original 87Sr/86Sr ratio can be tested by comparing the strontium, magnesium, or oxygen isotopes in the sample to modern counterparts.
| Sample Type | Recommended Sample Size |
| Forams (pre-extracted) | 10 mg |
| Shells, corals and carbonates | 100-150 mg |
| Marine Sediments | 50 mg |
See more information on Sample Types and Recommendations
Measurement
The strontium isotopes on samples are analyzed by first separating the strontium from the sample’s matrix through acid dissolution and ion-exchange chromatography. This separated strontium is then measured via mass spectroscopy and presented with a 95% confidence interval. This method works best over the past 600 million years (mainly from 0.15 to 500 Ma) with a maximum time resolution of 1 million years. (Read more on the Strontium Isotope analysis methods).
References
Antonelli, M.A., Pester, N.J., Brown, S.T. and DePaolo, D.J., (2017). Effect of paleoseawater composition on hydrothermal exchange in midocean ridges. Proceedings of the National Academy of Sciences, 114(47), pp.12413-12418.
Mearon, S., Paytan, A. and Bralower, T.J., (2003). Cretaceous strontium isotope stratigraphy using marine barite. Geology, 31(1), pp.15-18.
McArthur, J.M., Howarth, R.J. and Shields, G.A., (2012). Strontium isotope stratigraphy. The geologic time scale, 1, pp.127-144.
Peterman, Z.E., Hedge, C.E. and Tourtelot, H.A., (1970). Isotopic composition of strontium in sea water throughout Phanerozoic time. Geochimica et Cosmochimica Acta, 34(1), pp.105-120.
