Methodology: Strontium Isotope Analysis
Pretreatment
Low-blank separation of strontium from each sample achieved by extraction chromatography using Eichrom Sr resin following the methods of Pourmand et al., 2014 and Pourmand and Dauphas, 2010.
Additional option(s): strontium concentration for an additional fee only in conjunction with the isotope measurements
Sample types available for strontium (87Sr/86Sr) analysis: bones, foraminifera, igneous rocks, mineral dust, shells, corals, carbonates, tooth enamel, water and wool.
More information on Sample Types and Selection for strontium analysis.
87Sr/86Sr Measurement
Device: Neptune Plus multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) with an Apex-Q desolvation nebulizer or a self-aspirating nebulizer, depending on sample type and overall strontium concentration in the sample.
Results Reported
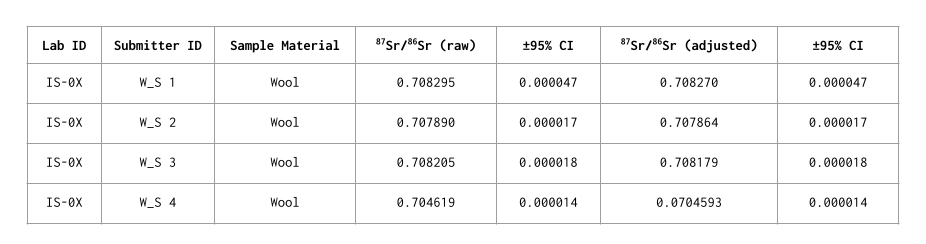
Strontium isotope ratio data are reported as 87Sr/86Sr ratios. The analytical accuracy and precision were assessed by multiple measurements of certified reference material (SRM 987) during the analysis. Uncertainties for each sample measurement is presented as the 95% confidence interval (CI).
Note: The lab offers strontium concentration for an additional fee only in conjunction with the isotope measurements.
Duration: 2 minutes, 9 seconds || Speaker: Arash Sharifi, PhD
This video excerpt is part of Isobar Science’s webinar: Geochemistry and Application of Strontium Isotopes.
Disclaimer: This video is hosted in a third-party site and may contain advertising.
References
Pourmand, A., Prospero, J.M. and Sharifi, A., (2014). Geochemical fingerprinting of trans-Atlantic African dust based on radiogenic Sr-Nd-Hf isotopes and rare earth element anomalies. Geology, 42(8), pp.675-678. DOI: 10.1130/G35624.1
Pourmand, A. and Dauphas, N., (2010). Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta, 81(3), pp.741-753. DOI: 10.1016/j.talanta.2010.01.008
Page last updated: November 2019
